The path to personalised hepatitis D treatment
Hepatitis D is by far the most severe form of chronic viral hepatitis frequently leading to liver failure, hepatocellular carcinoma and death. However, there is very limited knowledge on disease pathophysiology and host-virus interactions explaining the large interindividual variability in the course of hepatitis D. The D-SOLVE consortium ( (“Understanding the individual host response against Hepatitis D Virus to develop a personalized approach for the management of hepatitis D”), coordinated at MHH/CiiM, aims for an unbiased screening of a large multicenter cohort of well-defined HDV-infected patients to better understand individual factors determining the outcome of infection and to identify subjects benefitting from currently available treatments. In a highly competitive call for proposals, the D-SOLVE consortium has received four-year funding of 6.75 million euros from the Horizon 2020 EU Horizon Call „Personalised medicine and infectious diseases: understanding the individual host response to viruses (e.g. SARS-CoV-2)“ of the European Union.
More information can be found on the D-SOLVE project website.
Background

Hepatitis D is by far the most severe form of chronic viral hepatitis, frequently leading to liver failure, hepatocellular carcinoma and death. Hepatitis D is caused by coinfection Hepatitis D is caused by co-infection with hepatitis B virus (HBV) and hepatitis D virus (HDV). Up to 20 million individuals worldwide are infected with HDV including about 250.000 patients in the European Union. Hepatitis D is a major health burden in distinct regions in Central Asia and Africa but also a particular problem in some European countries such as Romania. Western and Central European countries have high HDV prevalence in immigrant populations, so an adequate cascade of care is a challenge.
There is still very limited knowledge about the pathophysiology of the disease and the interactions between host and virus, the latter being the reason for large interindividual variability in the course of HDV infection. Since Hepatitis D is an orphan disease in Western Countries, there have been no large multicenter cohorts of HDV-infected patients with appropriate biobanks. There is also no robust and reliable animal model available allowing to study distinct host responses. Finally, until recently there was no approved antiviral treatment option for hepatitis D. Pegylated interferon alfa has been used off-label with very limited success for almost two decades. The entry inhibitor bulevirtide received conditional EMA approval in August 2020 and is now available in some European countries, but numerous questions still remain about the optimal treatment. For example, it is not defined which patients should be given prioritized treatment, how long to treat and what to do with patients with suboptimal responses. The extremely high costs for this orphan drug are also a major barrier in several settings.
Thus, there is an urgent clinical, social and economic need to better understand individual factors determining the outcome of infection and to identify those individuals who will benefit from currently available treatment approaches. The D-SOLVE consortium is strongly convinced that Hepatitis D is a protype infectious disease which could benefit tremendously from a novel individualized infectious medicine approach based on a better understanding of the host immune response against HDV and identification of distinct immune biomarkers.
The D-SOLVE consortium hypothesize that comprehensive and unbiased screening of a large multicenter cohort of well- defined HDV-infected patients, followed by mechanistic studies to determine the functional role of distinct molecules will identify specific parameters and tools which can have an immediate impact to the management of patients with this most severe viral liver disease. Patient surveillance strategies and antiviral treatment approaches could be personalized which should reduce clinical and social disease burden, improve quality of life and safe direct and indirect costs caused by HDV infection.
Aim
The three key clinical questions in the management of hepatitis D which will be addressed by D-SOLVE are as follows:
- Viral control: It is completely unknown why some patients are able to control HDV infection with very low levels of HDV replication or even undetectable HDV RNA while others present with very high HDV viremia with HDV RNA levels above 10 million IU/ml. The percentage of HDV RNA negativity among anti-HDV positive patients ranges between 20-50%. Distinct factors being associated with viral control are largely undefined. Determining biomarkers indicating individual risks of viral flares and relapses is an urgent medical need for the daily management of hepatitis D patients.
- Disease progression: Chronic viral hepatitis leads to inflammation with subsequent development of liver fibrosis which can lead to liver cirrhosis. However, for all viral hepatitis infections it has been shown that fibrosis progression may take several years and sometimes even decades. Again, the factors that distinguish between benign and aggressive disease progression are largely unknown, especially in the early stages of infection. A deeper understanding of mechanisms that explain and predict the different outcomes would be highly desirable for the clinical management of hepatitis D patients. Optimal surveillance intervals and monitoring tools are not defined and could be based on new biomarkers. In addition, the decision to initiate potentially side-effect-prone and costly antiviral treatment approaches could be individualized based on clear individual risk profiles for long- term complications of liver disease.
-
Antiviral treatment response: Pegylated interferon alfa (PEG-IFNa) has been used (off-label) to treat HDV infection for almost two decades. However, PEG-IFNa treatment is associated with frequent and sometimes severe side effects and can lead to severe hepatitis flares in patients with HDV-associated autoimmune hepatitis, a particular common and sometimes serious feature of HDV infection. Moreover, reliable factors predicting response to PEG-IFNa treatment are not defined. Thus, there is an urgent need for personalized risk stratification which patients have realistic chance to benefit from PEG-IFNa therapy. In summer 2020, EMA granted a conditional approval for the treatment of hepatitis D to bulevirtide, a novel HBV/HDV entry inhibitor targeting the bile acid transporter NTCP, based on promising results from pivotal phase 2 studies. Bulevirtide, a peptide that requires daily s.c. injections, leads to a decline in HDV RNA levels and improvement of biochemical disease activity in the majority of patients without causing major systemic side effects. However, and importantly, several key questions how to use bulevirtide are completely unclear. E.g., once the entry inhibitor has been started, the optimal duration of treatment is undefined. While some patients may require life-long maintenance treatment, there is evidence that many patients may achieve immune control once the drug is stopped. There is concern, however, that severe relapses can lead to potentially life-threatening hepatitis flares if treatment is stopped too early. Therefore, biomarkers are needed to determine factors predicting long-term control of HDV after discontinuation of bulevirtide.
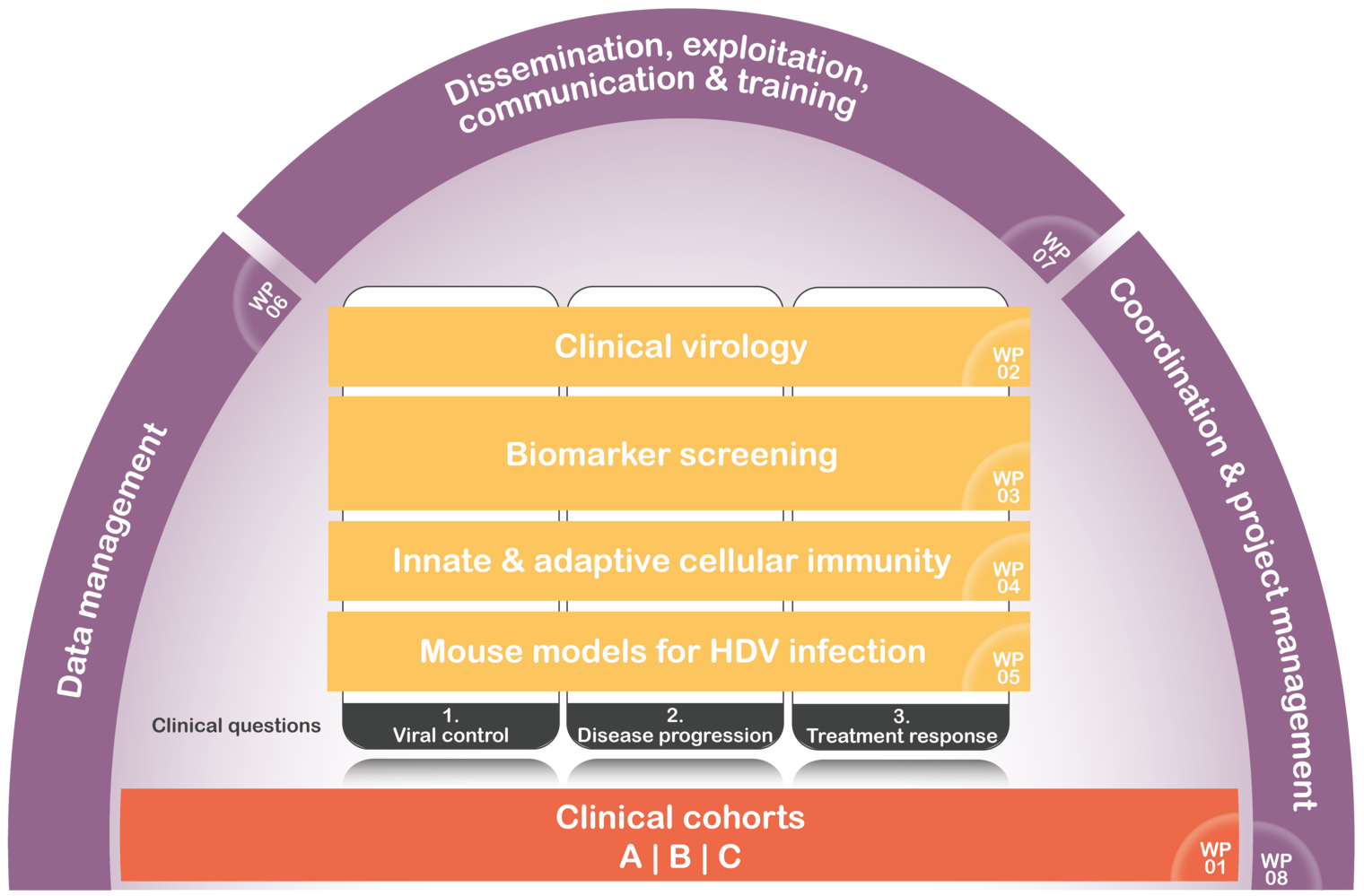
D-SOLVE will address these three key clinical questions across four translational research work- packages. We will investigate virological features of HDV infection, apply a broad and unbiased biomarker screening approach, study distinct innate and adaptive immune responses in the peripheral blood and the liver of HDV infected patients, and explore effects of identified molecules in mouse models – which could also lead to novel antiviral strategies.
Work Plan
In undertaking a highly challenging project as the present one, we planned a work schedule over a 48- month project period. This will ensure a maximum likelihood of success, eliminates or mitigates the risks inherent in reach of this nature, allowing continuous progress monitoring and review throughout the project lifecycle.
Interdependencies between the right research and development WPs will be ensured. To achieve the specific WP objective(s), the work plan activities are organized into eight Work Packages (WPs), each consisting of a number of associated tasks that collectively ensures the achievements of the project objectives. Progress against program, budget, and the obtained results will be monitored on a regular basis by the Project Steering Committee (PSC). Should it occur that any WP is late with respect to the programmed timing by more than one month, the Project Coordinator will bring it to the attention of the PSC, and a plan will be agreed and implemented to recover the delay.
In working towards personalized treatment of chronic hepatitis delta, the foundation of this project are large world-unique clinical cohorts (WP1) that will be utilized in upcoming WPs (2, 3, 4, and 5) in answering three key clinical questions on individual determinants of i) fibrosis progression, ii) HDV replication control, and iii) response to treatment. The project coordinator will produce the major periodic reports and minutes of progress meetings will give an update of the project progress at 6 monthly intervals during the program.
WP1 (Clinical cohorts) is the first work package starting at month 1 and lasting throughout the 48 months project period. This work package will lay the foundation for WPs 2 to 5 and in assessing the specific scientific questions on i) control of HDV replication, ii) individual determinants of fibrosis progression, and iii) treatment response. Three subprojects will be established with the first collecting in a cross-sectional approach 750 patients reflecting the entire spectrum HDV infection in Europe, the second consisting of a retrospective-prospective cohort of patients with liver biopsies taken 10-20 years ago to allow the investigation of intrahepatic immune correlates with disease progression and the third will be a prospective trial of patients being treated with the novel entry inhibitors bulevirtide.
WP2 (Clinical virology: testing for novel virological markers; HDV & HBV sequencing) starts at month 1 and lasts up to month 36. This work package will be essential to perform a comprehensive virological characterization of these patients who are infected with two viruses HBV and HDV. As local diagnostics in particular for HDV but also for novel HBV markers have not been fully standardized, central testing with optimized methodology will be required. In addition, WP2 will perform an in-depth viral sequencing of both HBV and HDV which will be crucial to determine immune pressure on the virus and to dissect host-viral interactions.
WP3 (Biomarker screening) starts at month 1 with retrospective samples (cohorts B and D) and will then continue with the other cohorts during the entire funding period until month 48. This work package will be the core project to identify novel biomarkers for the three key clinical challenges to be addressed in D-SOLVE. KI and HZI/CiiM will perform the respective screening in collaboration with MHH and INSERM.
WP4 (Innate and adaptive immunity) starts at month 1 and lasts up to month 48. This work package led by KI and MHH will explore mechanisms of biomarkers identified in WP3 but also focus on hypothesis-driven questions, in particular investigating intrahepatic immune innate and adaptive responses. Intrahepatic immune responses have rarely been studied in hepatitis D and thus completely novel data can be expected which can probably only generated within this D-SOLVE consortium combining translational clinical and basic expertise and providing the logistics to have access to the required patient material.
WP5 (Mouse models of HDV infection and HDV-induced transcriptomic and epigenetic modifications) starts at month 1 and lasts up to month 48. Given previous observations for HCV, it is likely that HDV control cure by either interferon-based therapies or in combination with novel antivirals like Bulevirtide will only partially eliminate HDV-induced modifications of the hepatocyte cell circuits or the innate and adaptive immune responses. These persistent perturbations of the liver cell circuits may contribute liver disease progression and HCC risk post cure. Supporting this concept, HDV induces DNA hypermethylation and histone modification in cell lines suggesting the existence of an epigenetic viral footprint relevant for viral pathogenesis and HCC risk. To address this question (1) we will study the HDV-induced transcriptomic and epigenetic modifications in liver tissues and PBMCs from cohorts of WP1 and study their phenotype before and after antiviral treatment. We will (2) then validate and characterize the modifications in well characterized animal models for HDV-HBV co-infection pre- and post-cure with antiviral therapies. (3) Finally, we will investigate the impact of these changes for progression of disease and cancer risk post cure. Through the understanding of these underlying mechanisms, this work program will enable the development of new biomarkers for treatment outcome, liver disease progression and HCC risk post cure.
WPs 6 (Data management), 7 (Dissemination, exploitation, IP, communication & training) and 8 (Coordination & project management) will be carried out along the whole duration of the project.
Partner
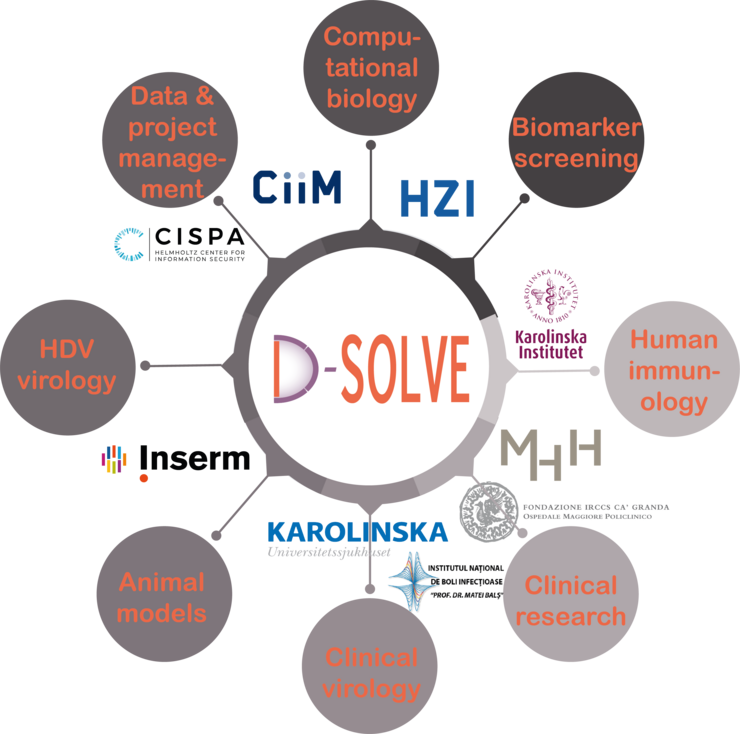
D-SOLVE aims to achieve individualised management of HDV by combining exceptional clinical, immunological, bioinformatical and virological expertise from leading centers in Europe. Several of the leading experts in European HDV research contribute to D-SOLVE. Moreover, clinical centers with an outstanding experience in translational research in Stockholm, Milan, Hannover and Bucharest follow some of the largest single center cohorts of patients with hepatitis D in Europe. Extraordinary high-level knowledge in performing an unbiased and broad biomarker screening from host genetics to host transcriptome and proteome analysis is present with high level expertise in computational biology. Mechanistic studies to investigate distinct molecules and pathways will be performed using novel vitro and animal models by foremost specialists in human immunology, HDV immunity and viral hepatitis research. Finally, CISPA Helmholtz Center for Information Security enables concepts for secure data exchange between centers.
Hepatitis D: B Aware
The D-SOLVE consortium is taking part in the "Hepatitis D: B Aware" campaign, which was launched on the occasion of World Hepatitis Day, which is celebrated every year on July 28. In short videos, researchers answer important questions about hepatitis D. CiiM director Prof. Marcus Cornberg explains the diagnosis of hepatitis D in his video.
Further videos from the campaign can be found in the TherVacB Youtube channel.
Funding

The D-SOLVE consortium is funded by the European Union in the Horizon Europe Framework Programme (HORIZON) under grant agreement No 101057917.
Partner
Prof Soo Aleman Karolinska University Hospital, Sweden
Prof Thomas Baumert INSERM, France
Prof Niklas Björkström Karolinska Institutet, Sweden
Prof Florin Caruntu National Institute for Infectious Diseases "Professor Doctor Matei Balș", Romania
Prof Pietro Lampertico Policlinico of Milan, Italy
Prof Yang Li HZI/CiiM, Germany
Dr Joachim Lupberger INSERM, France
Dr Yang Zhang CISPA, Germany
MHH/CiiM Collaborateurs
Prof Markus Cornberg MHH/CiiM, Germany
Dr Jennifer Debarry CiiM, Germany
Dr Helenie Kefalakes MHH, Germany
Dr Lisa Sandmann MHH, Germany
![[Translate to English:] Header Forschung [Translate to English:] Header Forschung](/fileadmin/_processed_/e/7/csm_AdobeStock_126036009_af86e9ae76.jpeg)