What is Individualised Medicine?
In medicine, the development and application of therapies with maximum efficacy and minimum side effects remains a fundamental challenge. In fact, approved therapies and medicines often show good efficacy in only a small proportion of patients, while other patients experience no improvement or even develop severe and sometimes dangerous side effects. In this context, the buzzword Individualised Medicine (often also called Personalised Medicine or Precision Medicine) has been causing lively discussions in research, clinical practice, industry and health policy for some years now.
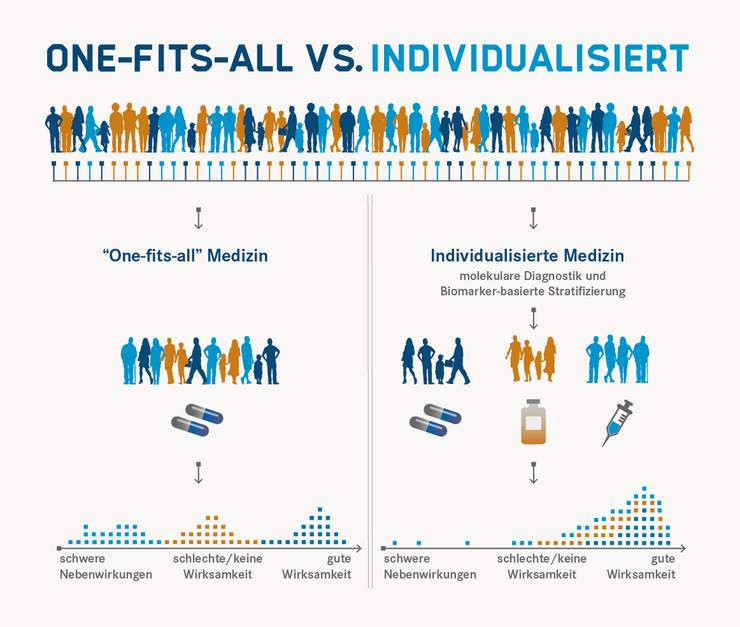
In Individualised Medicine, treatment is preceded by diagnostic tests, the results of which are intended to enable a therapy or prevention that is precisely tailored to the patient and the clinical picture. This not only increases effectiveness and safety for the individual patient, but also avoids unnecessary costs.
This basic idea is not entirely new. Since the very beginning, medicine has strived to capture the specifics of each individual patient in order to be able to treat them in the best possible and most individual way. Whereas medical experience has been an essential element for the personalisation of medicine in many areas up to now, new quantitative approaches are now emerging: In recent years, completely new technical methods have been developed in basic research that allow a comprehensive molecular analysis of each individual patient. So far, however, these methods have only been used to a very small extent in routine diagnostics. However, it can be seen that the comprehensive use of these new methods would already make it possible to raise diagnostics to a completely new level. For most approaches, only a simple blood sample is needed to create a detailed snapshot with the help of modern technologies that can provide information about molecular processes.
New paths in omics-based diagnostics
In addition to the study of the totality of genes in an organism - in short: genomics - the analysis of other biomolecules has also made enormous progress and can be applied in high-throughput procedures through automated processes. Transcriptomics provides doctors and life scientists with data on which genes are actually being read and to what extent. Proteomics reveals which different protein molecules occur as building blocks and biological catalysts in cells and tissues. Metabolomics records the totality of all metabolic products in the organism and glycomics provides information about existing protein modifications due to sugar components.
The aforementioned techniques, often summarised under the collective term "omics" due to their common ending, in interaction with increasingly powerful computer systems now make an enormous flood of data available. The challenge now is to process this data and link it with each other in such a way that predictions can be derived from it as precisely as possible. If this succeeds, the individual risks and susceptibilities of each person can be determined far more quickly and accurately than would have been thought possible just a few years ago.
Precise and evidence-based stratification of patients
It is not even necessary to understand what exactly the function of each gene or protein molecule is in order to draw conclusions from its presence. For practical medicine, it is often enough to know that a certain diagnostic result points to a disease, a particular risk or another important disposition. Ideally, the connection is simple: if a certain molecule can be detected in the body, disease X is present, or therapy Y is advisable. Such molecules - or other meaningful findings that can be detected by means of a diagnosis - are therefore called biomarkers. However, the detection of several different biomarkers is often necessary in order to be able to precisely predict complex disease processes. In addition, meaningful biomarkers are still lacking for many diseases.
With the help of biomarkers, but also other parameters (for example, age, medical history and family history, lifestyle and other physiological conditions), patients can be divided into groups for which certain therapy or prevention approaches can be useful. Experts call such a classification stratification. Stratification has also been practised in medicine for a long time. Individualised Medicine also aims to carry out this classification on the basis of exact, scientifically founded criteria and strives for the most precise stratification possible down to the individual. Thus, evidence-based stratification procedures that are as precise as possible are sought instead of making classifications only based on significantly more uncertain empirical values.
The future of individualised medicine has already begun
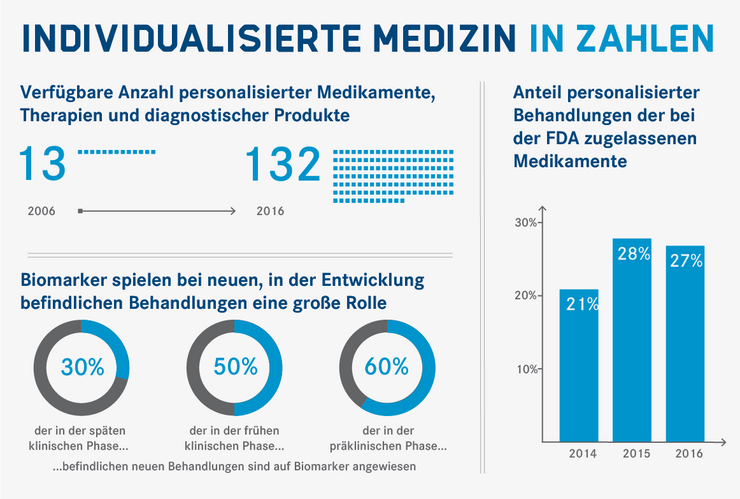
In some areas, the individualised approach is entering clinical practice and the use of biomarkers for targeted treatments is on the rise. The number of available personalised medicines, therapies and diagnostic products has increased many times over in recent years, and an ever-increasing percentage of treatments in development are based on biomarkers.
Cancer medicine in particular has developed strongly in the direction of individualisation in recent years. Today, tumour treatment in specialised centres is often preceded by a molecular genetic examination of the cancer cells, which allows a detailed stratification of disease subtypes. The characterisation of key mutations and molecular signalling pathways determines the diagnosis and subsequent therapeutic intervention tailored to the mutations found. For example, new antibody-based therapies against breast and colorectal cancer that are tailored to specific mutations have been astonishingly successful. But also in other disease fields, such as infectious medicine, therapies tailored to a specific patient group are increasingly being found. One of the most important drugs in HIV/AIDS therapy, for example, leads to severe, sometimes life-threatening side effects in some patients. This is due to a certain genetic predisposition. The presence of this predisposition (and thus an increased risk of corresponding side effects) is therefore checked by means of genetic tests before treatment in order to initiate an alternative therapy if this biomarker is present. In other cases, it is the pathogens instead of the patients that are divided into groups and combated with different methods. In the case of hepatitis C, for example, a different treatment is selected depending on the genetic type of virus present. These first examples of molecular and/or evidence-based stratification illustrate the importance and great potential of this approach and the individualised medicine it aims to achieve for all areas of medicine, not least infectious medicine.
Jennifer Debarry, 2018